41 fda approved health claims on food labels
CFR - Code of Federal Regulations Title 21 - Food and Drug ... One or more model health claims that represent label statements that may be used on a food label or in labeling for a food to characterize the relationship between the substance in a food to a... 'FDA Cleared' vs. 'FDA Approved': What's the Difference ... What the FDA does approve is specific products: food additives, drugs, certain medical devices, etc. "FDA Approved" means that the FDA, in its own words, "has determined that the benefits of the ...
Qualified Health Claims | FDA - U.S. Food and Drug ... Food manufacturers can petition the agency to consider exercising enforcement discretion for the use of a qualified health claim. The FDA does not "approve" qualified health claim petitions.
Fda approved health claims on food labels
Label Submission and Approval System (LSAS) | Food Safety ... The LSAS administrator will be your first contact. Email the administrator at LSAS@usda.gov or call 301-504-0837. We ask that you use these resources first rather than calling the main Labeling and Program Delivery Division (LPDD) lines so that we can track all issues and provide appropriate resolutions. "Drinking fluoridated water may reduce the risk of dental ... "Drinking fluoridated water may reduce the risk of dental caries" is _____ approved health claim that can be used on food labels. Question 32 options: an FDA an NLEA a USDA a DHHS Drinking fluoridated water may reduce the risk of dental caries is an FDA approved health claim that can be used on food labels. Label Claims for Food & Dietary Supplements | FDA Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient content claims,...
Fda approved health claims on food labels. CFR - Code of Federal Regulations Title 21 - Food and Drug ... (1) health claim means any claim made on the label or in labeling of a food, including a dietary supplement, that expressly or by implication, including "third party" references, written statements... CFR - Code of Federal Regulations Title 21 - Food and Drug ... (f) Model additional health claims for calcium and vitamin D. The following model health claims may be used in food labeling to describe the relationship between calcium, vitamin D, and... CBD, Label Claims, Food Safety Pose Potential Legal ... The FDA has essentially taken a hands-off approach, however, and focused its enforcement primarily on companies that make health claims around the benefits of CBD. Many companies in the food industry are hopeful the FDA will ease restrictions on CBD at some point, but Leongini said she doesn't think that is likely. FDA Food Product Labeling & Packaging ... - ESHA Research The Food Allergen Labeling and Consumer Protection Act of 2004 (FALCPA) mandates that packaged food items must declare, in plain language, the presence of any major food allergens (Milk, Egg, Fish, Crustacean shellfish, Tree nuts, Wheat, Peanuts, Soybeans, Sesame) on the product packaging.
Authorized Health Claims That Meet the Significant ... Authorized Health Claims That Meet the Significant Scientific Agreement (SSA) Standard Authorized health claims in food labeling are claims that have been reviewed by FDA and are allowed on food... All of the following are FDA approved health claims ... Weegy: Diets that contain plentiful sources of potassium may reduce the risk of high blood pressure and stroke.User: What type of claim must be supported by scientifically valid evidence for it to be approved for use on a food label ] Weegy: Health claim must be supported by scientifically valid evidence for it to be approved for use on a food label. Get the facts: Is it really 'FDA Approved'? The FDA does not approve individual food labels before food products can be marketed. But FDA regulations require specific labeling elements, including nutrition information, to appear on most ... CFR - Code of Federal Regulations Title 21 - Food and Drug ... PART 101 -- FOOD LABELING Subpart E - Specific Requirements for Health Claims Sec. 101.83 Health claims: plant sterol/stanol esters and risk of coronary heart disease (CHD). (a) Relationship...
Structure/Function Claims | FDA - U.S. Food and Drug ... If a dietary supplement label includes such a claim, it must state in a "disclaimer" that FDA has not evaluated the claim. The disclaimer must also state that the dietary supplement product is not... FDCA Expressly Preempts State-Law Claim Challenging ... The FDCA contains no express-preemption provision for drugs and the express-preemption provision for devices is worded differently than that applicable to food labeling. Nonetheless, Nacarino supports the proposition that the FDCA bars state-law claims based on truthful statements authorized by FDA regulations. So, while one should be mindful ... Label Claims for Conventional Foods and Dietary ... there are three ways in which fda exercises its oversight in determining which health claims may be used on a label or in labeling for a conventional food or dietary supplement: 1) the 1990... How to Read Food Labels: Understanding Claims & Components ... Reading a Food Label. The FDA has proposed some changes to the current nutrition facts label. We see both the old and the proposed new label here. ... For a health claim to be approved, it must be ...
CFR - Code of Federal Regulations Title 21 - Food and Drug ... The following model health claims may be used in food labeling to describe the relationship between diets that are low in saturated fat and cholesterol and that include soy protein and reduced risk...
FDA Issues Final Rule Clarifying Evidence of Off-Label ... The FDA's Final Rule also amends the regulations to provide that a company's intent that a product be used off-label may be shown not only by the company's "labeling claims, advertising ...
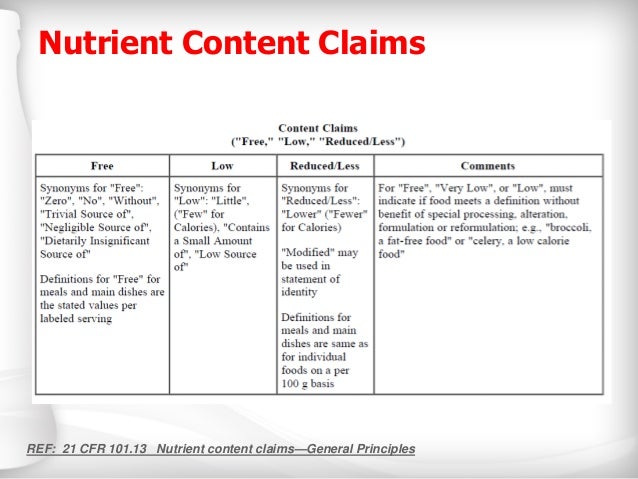
13 Misleading Food Label Claims and How Not to Be Tricked To use the label low-calorie in the United States, the FDA mandates that foods contain 40 calories or less per reference amount customarily consumed ( RACC ). Meals and main dishes should include 120 calories or less per 100 grams of food. 6. Label Says "Low-Carb" The FDA does not have any guidelines for the labeling of foods as low-carb.
Qualified Health Claims: Letters of Enforcement ... - FDA Contact FDA Follow FDA on Facebook Follow FDA on Twitter View FDA videos on YouTube Subscribe to FDA RSS feeds FDA Homepage Contact Number 1-888-INFO-FDA (1-888-463-6332)
CFR - Code of Federal Regulations Title 21 - Food and Drug ... The following model health claims may be used in food labeling to describe the relationship between noncariogenic carbohydrate sweetener-containing foods and dental caries. (1) Examples of the full...

34 Which Of The Following Claims Could Not Appear On A Supplement Label Without Fda Approval ...
The Dos and Don'ts of Dietary Supplement Labeling ... In February, FDA announced that it had recently sent warning letters to 10 supplement manufacturers who were claiming that their products could treat or cure depression and other mental health disorders. No dietary supplement, no matter how effective it is, may contain labeling that claims to "diagnose, mitigate, treat, cure or prevent" disease.
Fda Food Label - 16 images [Fda Food Label] - 16 images - new u s fda food labeling rules youtube, fda new food labeling regulations and compliance 2017, in a new aisle energy drinks sidestep some rules the new york times, three steps to plan for the fda s new food label rules 2016 10 18,
FDA Warns Companies to Stop Making False Claims for THC ... The Food and Drug Administration (FDA) has issued a warning to five companies that have been illegally selling THC and CBD products, falsely claiming they can diagnose, cure, prevent, alleviate or ...
Nutrient Content Claims - FDA Nutrient Content Claims. See Claims That Can Be Made for Conventional Foods and Dietary Supplements for definitions of claims. Final Rule: Food Labeling: Nutrient Content Claims; Alpha-Linolenic ...
Drinking fluoridated water may reduce the risk of dental ... Drinking fluoridated water may reduce the risk of dental caries is _____ approved health claim that can be used on food labels. Question 26 options: an FDA an NLEA a USDA a DHHS Drinking fluoridated water may reduce the risk of dental caries is an FDA approved health claim that can be used on food labels.
Label Claims for Food & Dietary Supplements | FDA Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient content claims,...
"Drinking fluoridated water may reduce the risk of dental ... "Drinking fluoridated water may reduce the risk of dental caries" is _____ approved health claim that can be used on food labels. Question 32 options: an FDA an NLEA a USDA a DHHS Drinking fluoridated water may reduce the risk of dental caries is an FDA approved health claim that can be used on food labels.
Label Submission and Approval System (LSAS) | Food Safety ... The LSAS administrator will be your first contact. Email the administrator at LSAS@usda.gov or call 301-504-0837. We ask that you use these resources first rather than calling the main Labeling and Program Delivery Division (LPDD) lines so that we can track all issues and provide appropriate resolutions.


![Food Labeling 101 - FDA Regulations Guide [2021] | Artwork Flow](https://uploads-ssl.webflow.com/5f59aa263c234bb74025de57/5fa4f8a355c6935dd2dde09d_Inner-Images-1.jpg)


Post a Comment for "41 fda approved health claims on food labels"